Compounds Worksheet Writing Ionic Formulas – Ionic compounds are a form of chemical compound which consists by positively charged and charged ions, or cations, as well as negatively charged ions. They are also called anions. They are formed by transfer of electrons from one element to the next creating a bond that connects the two. In this section we’ll discuss the features of ionic compound and the process by which they form.
Chemical Bonds in Ionic Compounds
Ionic compounds are bonded through ionic bonds. Ionic bonds are a type of chemical bond that results from the attraction between oppositely charged Ions. These bonds are extremely strong and possess high melting and boiling points. The exchange to electrons by cations as well as anions creates a net charge in the compound which is balanced by the crystal lattice structure. In this article, we will discuss the various types of chemical bonds and the properties of ionic bonds and the methods by which they’re made.
Cations, Anions, and Polyatomic Ions
Positively charged ions are referred to as Cations, while anions are ions that have a negative charge. They are formed by atoms losing or gaining electrons to attain a stable electron configuration. Polyatomic ions consist of an atom or two that are in a covalent relationship and have their own net charge. In this section, we’ll describe and present examples of cations, anions, and polyatomic Ions.
Writing Formulas for Ionic Compounds
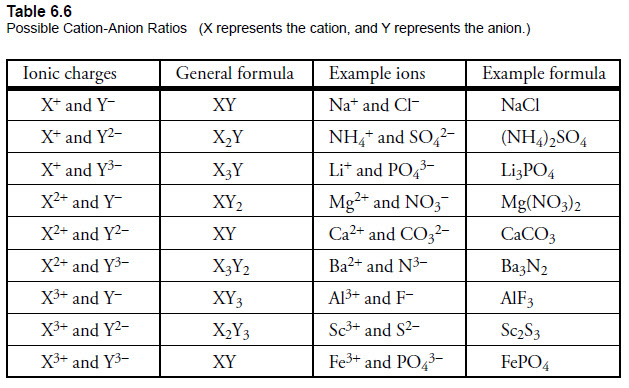
Writing formulas for ionic compounds involves identifying the cation and anion, and then making use of their charges to offset the charge of the compounds. There are certain guidelines to follow when formulating formulas for Ionic compounds. In the case of binary ionic compounds the charge of the cation is first expressed, followed by that of the anion’s. The charges are then used to determine the appropriate subscripts to balance the compound’s charge. In the case of polyatomic ionic compounds charges of the polyatomic ion are employed exactly the same way. In the following sections, we will explain how to formulate formulas for binary and polyatomic compounds as well as challenges to practice this ability.
Naming Ionic Compounds
Naming Ionic compounds is about identifying the cation and anion and using their names in order to form names for the compounds. For binary Ionic compounds, the cation’s name is first written, followed by the anion’s name and the ending is changed to “-ide.” In the case of polyatomic ionic compounds you will find the name for the anion is used. In this section we will discuss the guidelines for naming ionic compounds and provide examples of naming biatomic and polyatomic ionic compounds and offer exercises for improving your naming skills.
Properties of Ionic Compounds
Ionic compound have unique chemical and physical properties they can be utilized in numerous applications. They have high melting and boiling points, are brittle as well as being excellent conductors electricity when dissolved in water or melting. They are widely used in industrial processes and in everyday things like table salt and baking soda. In this section it will be discussed the physical and chemical characteristics of ionic compounds, as well as their many uses.
In the end, our Ionic Compounds Worksheet covers the essential topics related to ionic compounds, such as writing formulas, naming compounds, and knowing their properties. With practice and examples this worksheet makes great for Chemistry students who are looking to improve their abilities and understanding of the ionic compounds.