Arranging Ionic Compounds In Order Of Increasing Energy Worksheet – Ionic compounds are one type of chemical compounds that are made up in positively charged ions, or cations. Also, they contain negatively charged ions, or anions. They are formed through the transfer of electrons between elements that results in a bond in between two of the ions. In this article we’ll discuss the specifics of ionic compounds and how they’re formed.
Chemical Bonds in Ionic Compounds
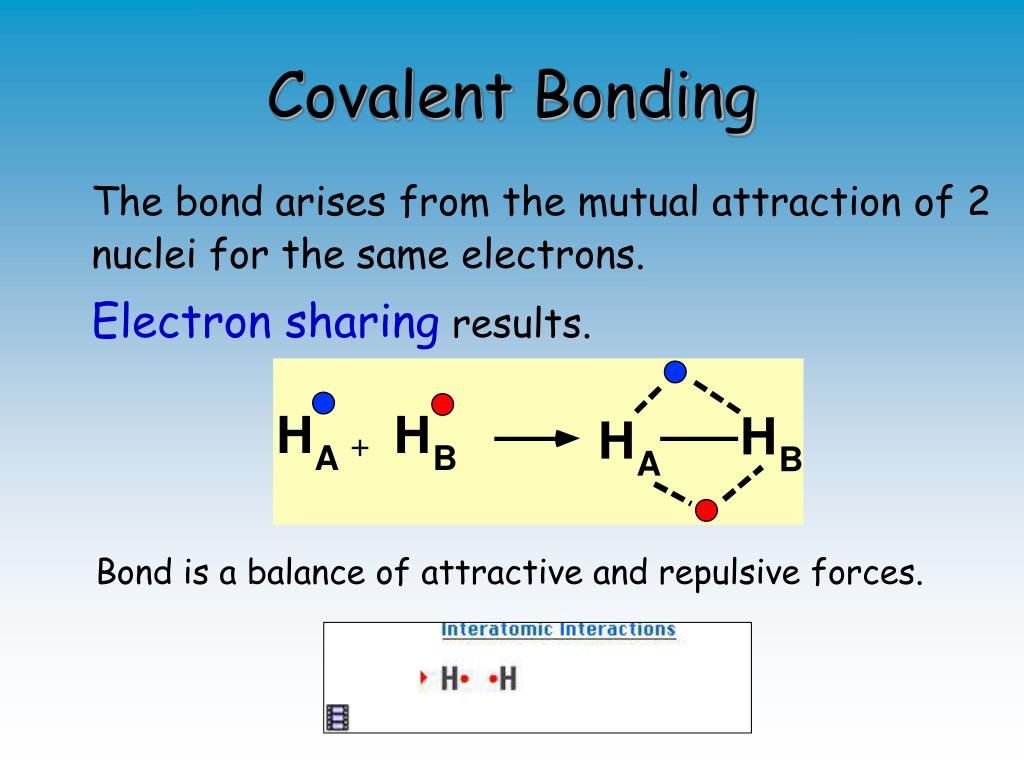
Ionic compounds are linked through ionic bonds. Ionic bonds are a type of chemical bond that arises due to the attraction between opposing charged Ions. Ionic bonds are very durable with high melting and boiling points. The exchange in electrons among cations and anions causes an increase in the charge of the compound that is balanced by the crystal’s structure. In this section this article, we’ll go over the various types of chemical bonds which are formed, the characteristics of ionic bonded and how they’re created.
Cations, Anions, and Polyatomic Ions
Positively charged ions are referred to as Cations, while anions are negatively charged ions. These ions form by atoms losing or gaining electrons in order to maintain the stability of their electron configuration. Polyatomic ions are ions that comprise many atoms covalently bonded together and have an electric charge. In this article, we will define and provide examples of anions, cations, and polyatomic Ions.
Writing Formulas for Ionic Compounds
Formulating formulas based on ionic compound requires identifying the cation as well as anion and using their charges to balance the compound’s charge. There are certain rules that must be followed when writing formulas pertaining to ionic compounds. For binary ionic compounds the cation’s charge is first written, then followed by the anion’s charge. The charges are used to determine which subscripts are required to balance the charge of the compound. For polyatomic ionic compounds, charges from the polyatomic element are utilized exactly the same way. Here, we’ll show examples of how you can write formulas for binary and polyatomic ionic compounds . We will also provide practical problems to master this process.
Naming Ionic Compounds
Naming ionic substances involves making sure that the anion is identified as well as the cation and the use of their names for the compound’s name. For binary ionic compound, the name of the cation is written first, then the anion’s name and the ending is changed to “-ide.” For polyatomic ionic compounds, it is the name given to the ion is used. In this section we will explain the procedures for naming Ionic compounds give examples of the naming of the polyatomic and binary ionic compounds and offer exercises for you to sharpen your naming skills.
Properties of Ionic Compounds
Ionic compounds have distinct physical and chemical properties which make them suitable for numerous applications. They have high melting and boiling points, are hard, and are excellent conductors of electricity when they are dissolved in water or melting. They are extensively used in industrial processes and in everyday items like table salt and baking soda. In this article we will explore the physical and chemical characteristics of Ionic compounds as well as their numerous applications.
In the end, our Ionic Compounds Worksheet will help you understand the key topics related to ionic compounds. This includes formulas to write formulas, naming compounds, and knowing their properties. With examples and exercises this worksheet provides an excellent resource for Chemistry students who want to enhance their skills and knowledge about Ionic compounds.