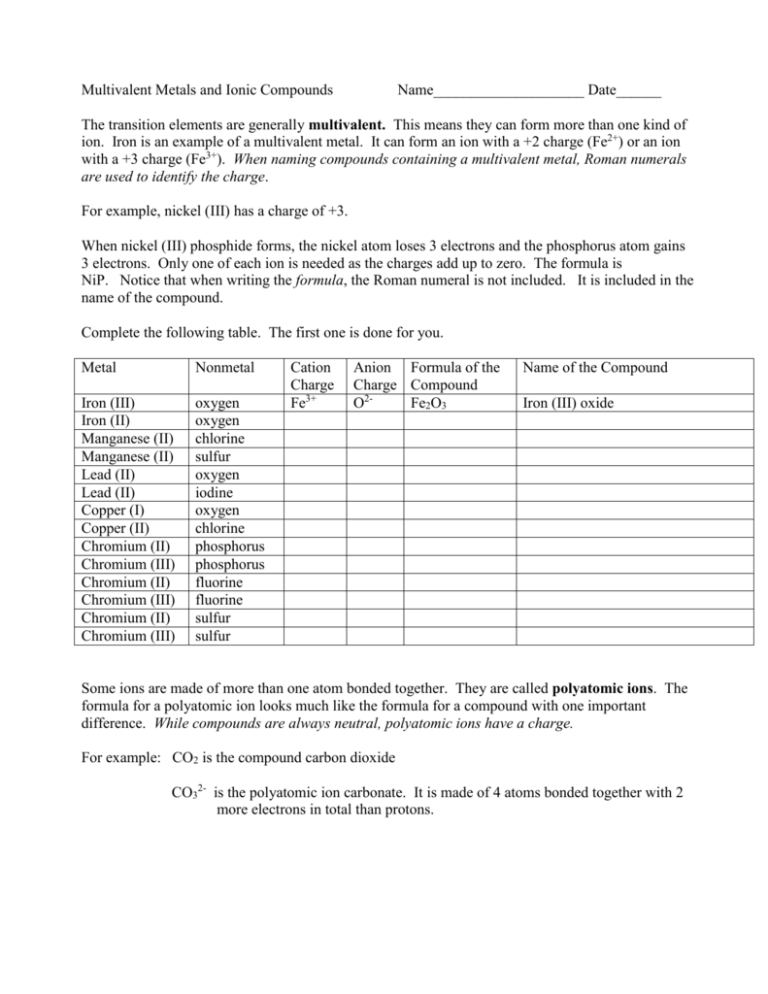
Ionic Compounds With Multivalent Metals Worksheet – Ionic compounds are the most common type of chemical compound which consists with positively charged particles, or cations. They also contain negatively charged ions. These are known as anions. They are created through the transfer of electrons from one element to the next and create a bonds in between two of the ions. In this article we’ll discuss the specifics of ionic compounds and how they are formed.
Chemical Bonds in Ionic Compounds
Ionic compounds are linked by ionic bonding, which are a kind of chemical bond resulting due to the attraction between opposing charged Ions. Ionic bonds are very durable and possess high melting and boiling points. The exchange of electrons between cations as well as anions creates a net charge in the compound, which is balanced out by the crystal’s crystal lattice. In this article we’ll look at how chemical bonds are formed and the properties of Ionic Bonds and the way they are made.
Cations, Anions, and Polyatomic Ions
The ions that are positive charge while anions are ions that have a negative charge. These ions form by atoms losing or gaining electrons in order to maintain an stable electron configuration. Polyatomic ions are composed of the presence of two or more molecules that are tightly bonded and have a net charge. In this section, we’ll define and provide examples of anions, cations and polyatomic Ions.
Writing Formulas for Ionic Compounds
Formulating formulas for Ionic compounds involves identifying the cation and anion and using their charges to calculate the charge of the compound. There are specific rules that must be followed when formulating formulas for Ionic compounds. For binary ionic compounds the charge of the cation is first expressed, followed with the charge of anion. The charges are used for determining the subscripts necessary to balance the compound’s charge. Polyatomic ionic compounds charges of the polyatomic isotope are utilized exactly the same way. This section we’ll provide examples of how write formulas for binary and polyatomic ionic compounds . We will also provide problem-based exercises for mastering this aptitude.
Naming Ionic Compounds
Naming ionic compounds is the process of identifying the cation and anion and making use of their names to make an ionic compound’s name. In the case of binary ionic compounds the cation’s name is first written, after which the anion’s is written and the ending is changed to “-ide.” When it comes to polyatomic ionic compound, names of polyatomic Ion is used. In this section we will go over the rules of naming Ionic compounds as well as examples of how to name binary and polyatomic ionic compounds as well as provide exercises to improve your name-naming skills.
Properties of Ionic Compounds
Ionic compound have unique physical and chemical characteristics that are useful in a variety of applications. They possess high boiling and melting points, they are brittle and are good conductors of electric current when they are submerged in water or melted. They are frequently used in industrial processes, and within everyday items such as table salt and baking soda. In this section, we will discuss the chemical and physical characteristics of ionic compounds, as well as their diverse applications.
In the end the worksheet on Ionic Compounds covers the essential topics related to ionic compounds, including formulas for writing formulas as well as naming compounds and knowing their properties. With examples and exercises this worksheet makes an excellent reference for chemistry students looking to improve the skills of and understand the ionic compounds.