Lewis Dot Ionic Compounds Worksheet – Ionic compounds are a type of chemical substance that consists of negatively charged ions, called cations, and negative charged ions. These are known as anions. They are formed via the transfer of electrons between elements and create a bonds that connects the two. In this article we will explore the specifics of ionic compounds and the process by which they form.
Chemical Bonds in Ionic Compounds
Ionic compounds can be held together by ionic bonds, which are a form of chemical bond which results from the attraction between oppositely charged ions. They are extremely durable as well as having high melting and boiling points. The exchange to electrons by cations as well as anions causes an added charge to the compound which is balanced by the crystal’s structure. In this article, we will discuss how chemical bonds are formed and the properties of Ionic Bonds and the ways in which they’re created.
Cations, Anions, and Polyatomic Ions
Citons are positively charged, while anions are ions that have a negative charge. These ions form by atoms losing or gaining electrons to achieve an ideal electron configuration. Polyatomic ions are composed of at least two atoms that are covalently bound and possess an average charge. In this section, we will define and provide examples of cations, anions, and polyatomic ions.
Writing Formulas for Ionic Compounds
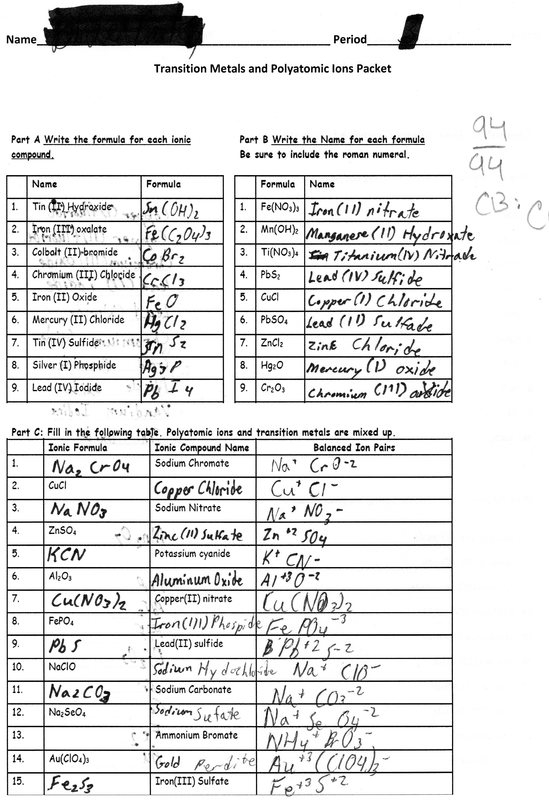
Formulating formulas based on ionic compound requires identifying the cation as well as anion and using their charges to calculate the charge of the compound. There are certain rules that must be followed when formulating formulas for Ionic compounds. For binary ionic compounds, the cation’s charge will be first written. It will then be followed by the anion’s charge. The charges are used to determine the subscripts that are needed to balance the compound’s charge. For polyatomic-ionic compounds charges of the polyatomic ion can be used similarly. Here, we’ll provide examples of how to formulate formulas for binary and polyatomic ionic compounds . We will also provide questions to practice the capability.
Naming Ionic Compounds
Naming ionic compounds involves making sure that the anion is identified as well as the cation and applying their names to form what is known as the chemical’s title. For binary ionic compound, the name of the cation is first written. It is followed by the anion’s with the end being changed to “-ide.” In the case of polyatomic ionic compounds the name of the polyatomic anion is used. In this section we will explain the rules of naming Ionic compounds include examples of naming the polyatomic and binary ionic compounds and also offer exercises for you to sharpen your naming skills.
Properties of Ionic Compounds
Ionic compounds have distinct chemical and physical properties which make them suitable for many different applications. They have high melting and boiling points, are brittle they also conduct electricity when dissolved in water or melting. They are typically used in industrial processes as well as in everyday items like table salt and baking soda. In this article we will look at the physical and chemical characteristics of these compounds and their many uses.
In conclusion our worksheet for Ionic Compounds provides the most important topics related to ionic chemicals, such as formulas to write formulas, naming compounds, and understanding their properties. With examples and practice problems this worksheet can be the perfect resource for students looking to expand their skills and understanding of the ionic compounds.