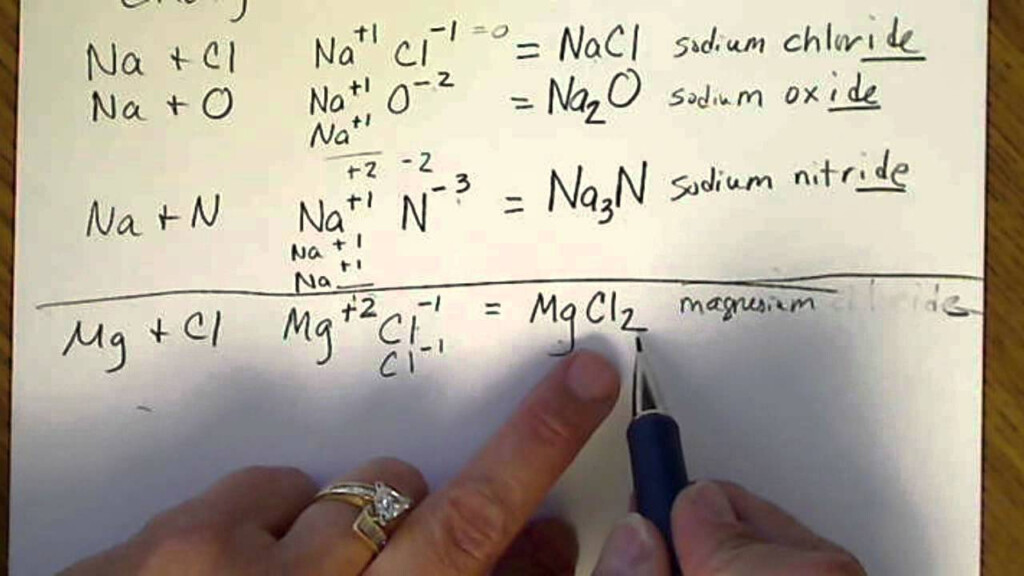Naming And Writing Formulas For Ionic And Molecular Compounds Worksheet – Ionic compounds are a form of chemical compound which consists in positively charged ions, or cations. Also, they contain negatively charged ions. They are also called anions. They are formed via the transfer of electrons from one element to the next leading to a bonded with the two particles. In this article, we will discuss some of the characteristics of these compounds and how they’re formed.
Chemical Bonds in Ionic Compounds
Ionic substances are joined via ionic links, which are a kind of chemical bond , which arises due to the attraction between opposing charged ions. They are extremely strong with high melting and boiling points. The exchange of electrons between cations and anions results in net charge for the compound, which is balanced out with the crystal’s complex lattice. In this section we will look at the various kinds of chemical bonds and the properties of ionic bonds and the way they are created.
Cations, Anions, and Polyatomic Ions
Cations are positively charged ions, while anions are ions that have a negative charge. They are formed by atoms losing or gaining electrons to form stabilised electron configuration. Polyatomic ions comprise many atoms in a covalent relationship and have the charge of a net. In this section, we’ll be defining and illustrating Cations, Anions, and polyatomic ions.
Writing Formulas for Ionic Compounds
Formulating formulas to describe ionic compounds requires identifying the cation as well as anion, and then using their charges to calculate the charge of the compound. There are specific rules that must be followed in formulas to write for ionic compounds. In the case of binary compounds, the charge of the cation must be written first, then to the anion’s cost. The charges are used to determine the subscripts needed to balance the charge of the compound. For polyatomic ionic compounds, the charges of the polyatomic element are utilized in the same way. In the following sections, we will show examples of how you can write formulas for binary and polyatomic Ionic compounds. We will also offer questions to practice the aptitude.
Naming Ionic Compounds
Naming ionic substances involves an identification of the anion and cation and creating their names as their names. In the case of binary ionic compounds the cation’s name is first written, then followed by the anion’s with the ending changing to “-ide.” In the case of polyatomic ionic compounds they are named after the polyatomic Ion is used. In this section we will review the procedures for naming Ionic compounds we will provide examples of naming those with polyatomic as well as binary ionic properties and also provide practice problems to improve your name-naming skills.
Properties of Ionic Compounds
Ionic compounds possess unique chemical and physical properties which make them suitable for many applications. They possess high boiling and melting points, and are brittle and they are excellent conductors of electric current when they are submerged in water or melting. They are used extensively in industrial processes, as well as within everyday items such as baking soda and table salt. In this article we will go over the physical and chemical characteristics of these compounds and their numerous uses.
In conclusion, our Ionic Compounds Worksheet will cover the fundamental topics related to ionic chemicals, such as formulas for writing formulas as well as naming compounds, and knowing their properties. With examples and practice problems This worksheet is an excellent tool for students seeking to develop their knowledge and skills in the ionic compounds.