Naming Compounds Ks3 Worksheet – Naming compounds is the most fundamental idea in chemical science. It involves assigning a unique name to chemicals based on its composition. An individual’s name on a chemical compound is a crucial indicator of its properties and the structure. There are many types of chemical compounds. These include covalent compounds, ionic compounds the binary type of compounds.
Naming Ionic Compounds
Ionic compounds are produced by moving electrons around atoms. They are composed in positively charged caustics and negatively charged anions. The rules for naming ionic compounds are as follows:
- Inscribe the name of compound first, and then an anion’s name.
- If the cation may have multiple possible charges, indicate the charge using Roman numbers in parentheses.
- In the case of a multiatomic ion make use of the name for the anion.
Examples:
- NaCl is the name given to sodium chloride.
- FeCl3 is also known as iron(III) chloride.
- Mg(NO3)2 is also known as magnesium nitrate.
Naming Covalent Compounds
Covalent compounds arise from the exchange of electrons between atoms. They are composed of molecules made consisting of two or even more atoms. The guidelines for naming covalent compounds are as like this:
- Inscribe the name of the first element in the formula.
- Enter“ide” in place of “ide” of the formula, changing the end“ide “-ide”.
- Prefixes indicate the quantity of atoms contained in each element in the molecule, with“mono” as a prefix “mono-” for the first element.
Examples:
- CO2 is the name given to carbon dioxide.
- N2O is named dinitrogen monoxide.
- The name SF6 refers to sulfur hexafluoride.
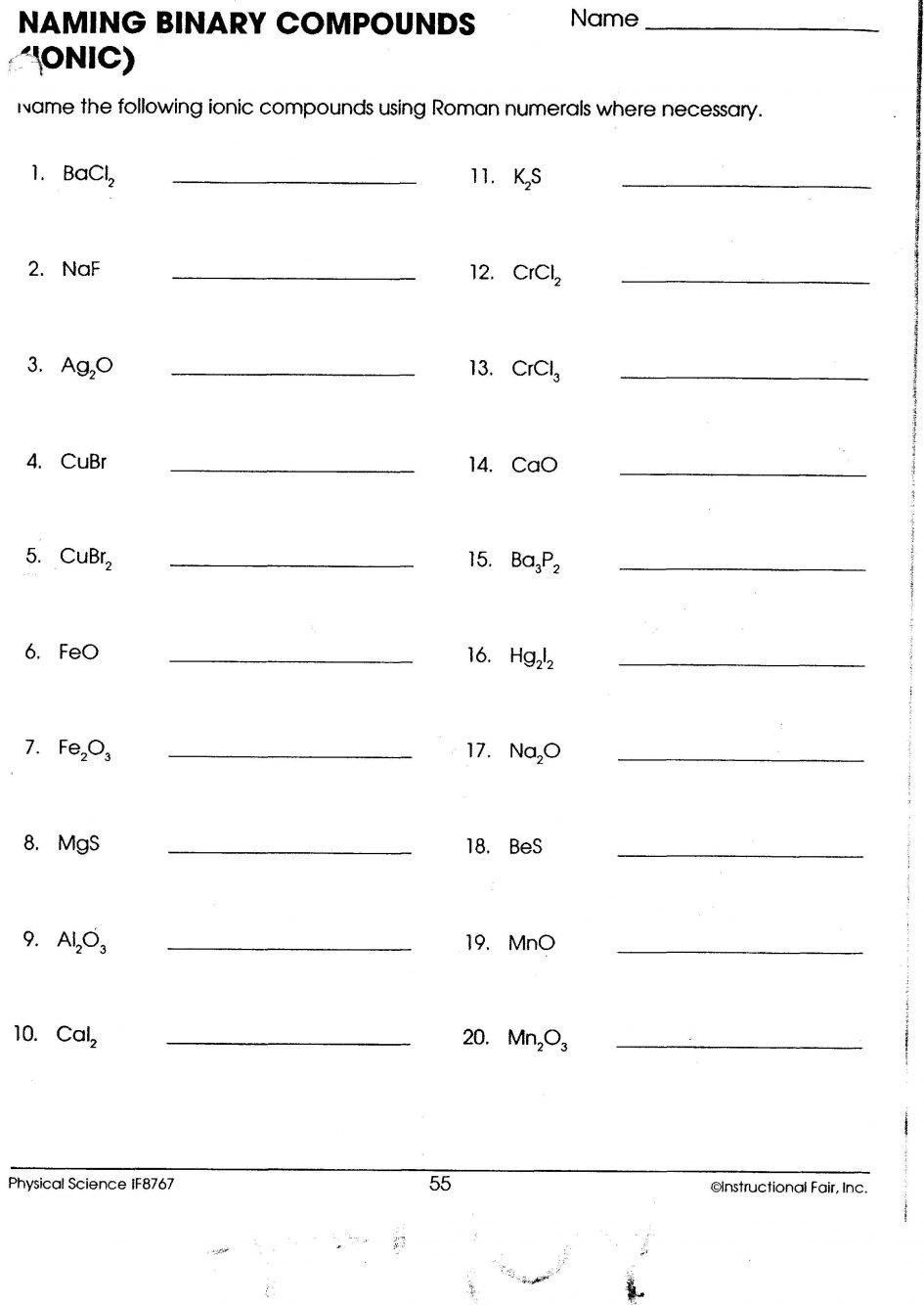
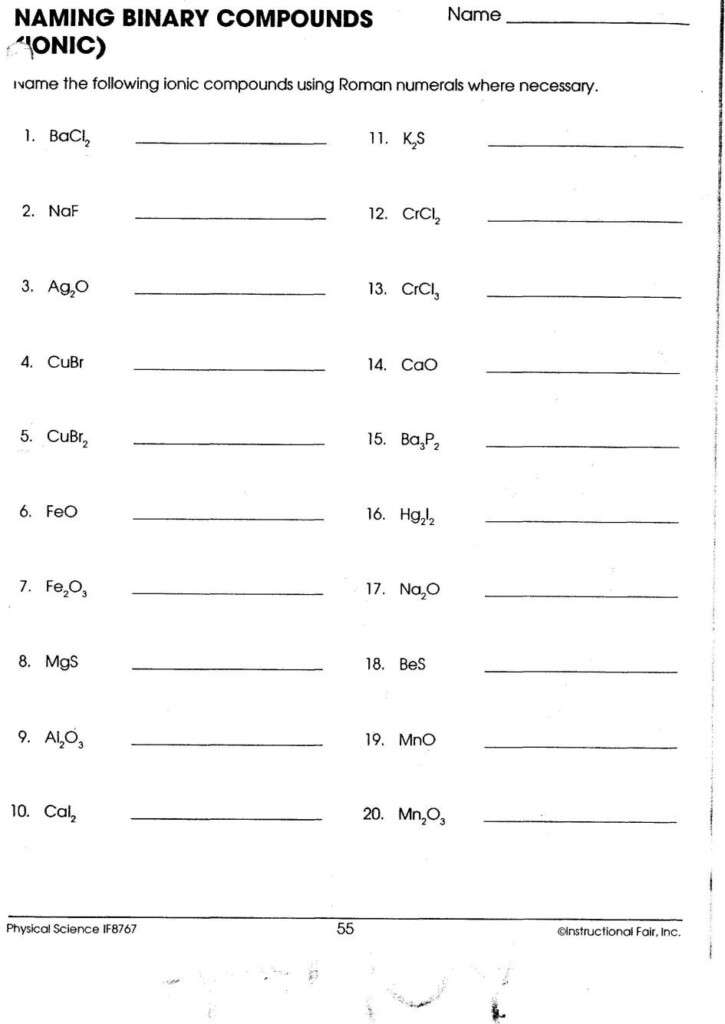
Naming Binary Compounds
Binary compounds are compounds made from two elements. The rules for using the term binary compound are as according to:
- Write the name of the first element of the formula.
- Enter“I” as the title of your second ingredient of the formula, changing the end“ide “-ide”.
Examples:
- HCl is named hydrogen chloride.
- CO is also known as carbon monoxide.
- CaO is the term used to describe calcium oxide.
Practice Exercises
In order to reinforce the learning process it will be accompanied by drills for naming Ionic elements, covalent components, or binary substances. The exercises will assist students to gain a thorough understanding of what rules are used for naming chemical compounds.
Ionic Compound Naming Exercises:
- Na2S
- KBr
- CaF2
- Al2O3
Covalent Compound Naming Exercises:
- CO
- SO2
- N2O4
- H2O2
Binary Compound Naming Exercises:
- Cl2O7
- P2S5
- BrF3
- NO
By completing these exercises, students will develop confidence in naming chemical compounds and will be able to apply these rules to other chemical compounds.
Conclusion:
Naming compounds is a crucial concept in the field of chemistry. It requires a deep understanding of how to follow the guidelines and rules for giving different compounds different names. Following the guidelines laid out in this worksheet, and working using the activities included, students will be able quickly identify covalent, ionic the binary chemical compounds. This knowledge is essential for success in chemistry and lays the foundation for further research in the field.