Naming Covalent Compounds Type Iii Worksheet 5 1 – Naming compounds is a key idea in chemistry. It involves the assignment of a unique name to an chemical compound, based on its composition. When you name a chemical compound provides crucial information about its properties as well as its structure. There are different types of chemical compounds, including organic compounds, covalent ones, or binary substances.
Naming Ionic Compounds
Ionic compounds are formed through electron transfer between electrons. They are composed of positively charged cations and negatively charged anion. The rules used to name ionic compounds are as the following:
- Write the name for the cation first. Then, write its name.
- If the cation is charged with more than one charge be sure to identify the charge using Roman numerals within parentheses.
- If an anion’s structure is polyatomic Ion, use the name of the anion.
Examples:
- NaCl is a name for sodium chloride.
- FeCl3 is named iron(III) chloride.
- Mg(NO3)2 is known under the name magnesium nitrate.
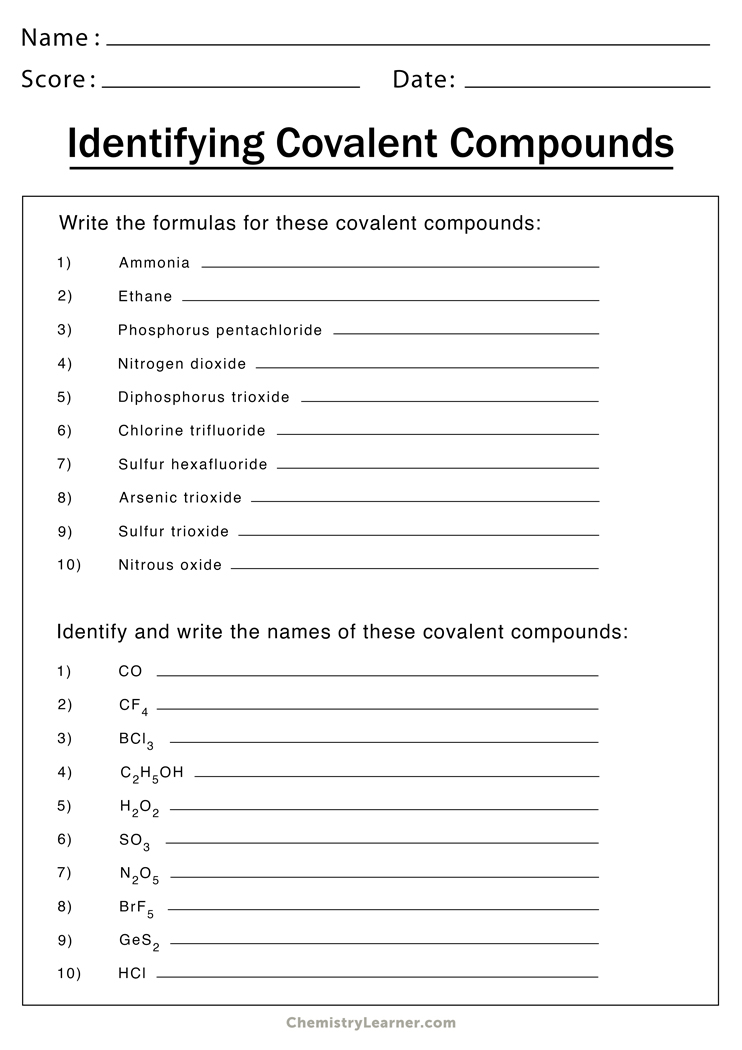
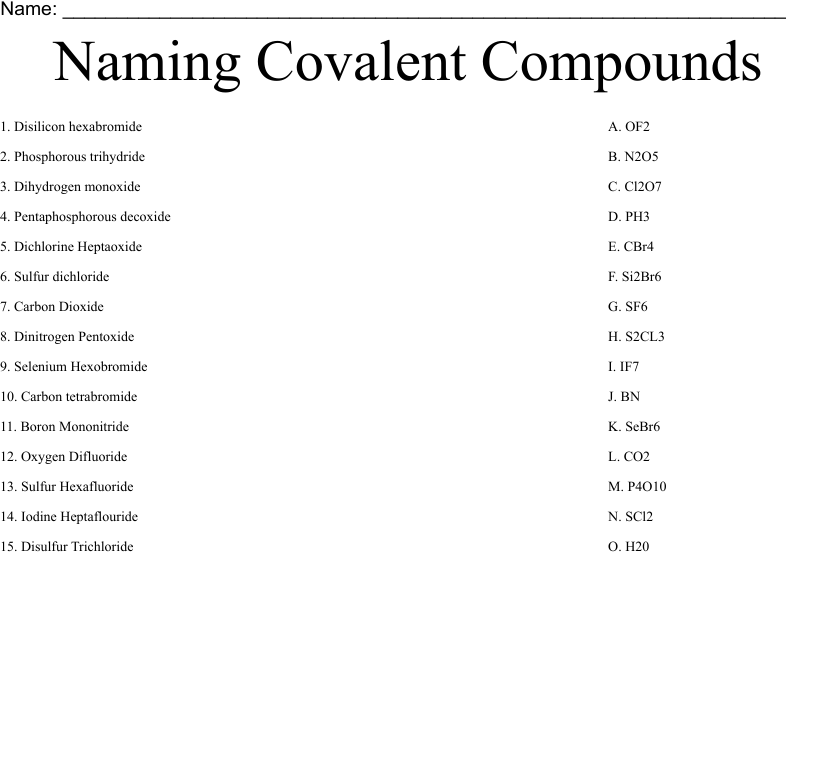
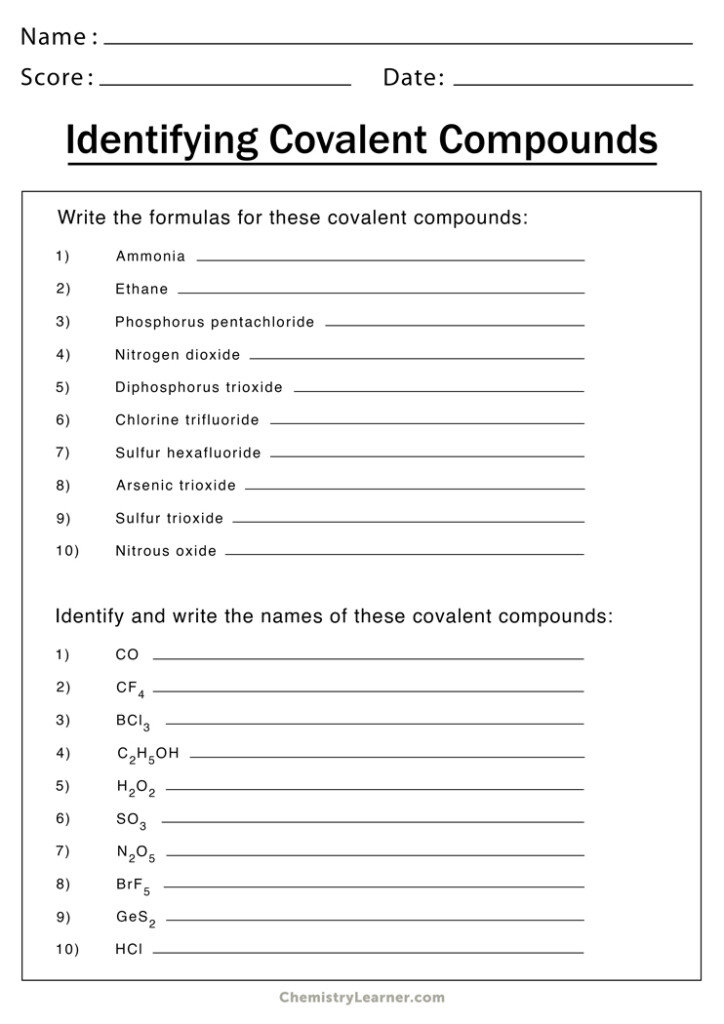
Naming Covalent Compounds
Covalent compounds arise from the exchange of electrons between atoms. They are made up of molecules composed comprising two or more atoms. The guidelines for naming covalent compounds are as in the following order:
- Then write the name of first element of the formula.
- Write your name for the element in the formula, changing the end“-ide” to “-ide”.
- Utilize prefixes to represent the number of atoms present in each element in the molecule. The exception is“mono-,” the particular prefix “mono-” for the first element.
Examples:
- CO2 is also known as carbon dioxide.
- N2O is named dinitrogen monoxide.
- This is known as sulfur hexafluoride.
Naming Binary Compounds
Binary compounds are made of two components. The rules for using the term binary compound are as like:
- Write the name of the first element in the formula.
- Write“double element” of the formula, changing the end“-ide. “-ide”.
Examples:
- The chemical name for HCl is hydrogen chloride.
- CO is a chemical compound known as carbon monoxide.
- CaO is the name given to calcium oxide.
Practice Exercises
To further reinforce the learning The worksheet will provide the practice of naming ionic and covalent substances, as well as binary compound. These exercises will allow students to achieve a good understanding of how to name chemical compounds.
Ionic Compound Naming Exercises:
- Na2S
- KBr
- CaF2
- Al2O3
Covalent Compound Naming Exercises:
- CO
- SO2
- N2O4
- H2O2
Binary Compound Naming Exercises:
- Cl2O7
- P2S5
- BrF3
- NO
Through these exercises, students will gain confidence in understanding chemical compound names and be able to apply the rules to other compounds.
Conclusion:
Naming compounds is an essential notion in chemistry and requires a clear understanding of how to follow the guidelines and rules to Naming different kinds of compounds. Following the guidelines outlined in this worksheet, and working by using the included exercises, students will be able to successfully identify ionic, chemical or binary compound. This information is crucial to achievement in chemistry. It will also provide solid foundations for further research in the field.