Naming Covalent Compounds Worksheet And Answers – Naming compounds is a basic concept in the field of chemistry. It involves granting a unique name to the chemical compound on the basis of its composition. In addition, the name assigned to the compound provides important information about its properties and structures. There are various types that chemical compounds can be found, including ionic compounds, covalent compounds also known as binary compounds.
Naming Ionic Compounds
Ionic compounds are created by the transfer of electrons between atoms. They consist made up of positively charged anion as well as negatively charged anion. The rules for naming Ionic compounds are as the following:
- Write the name for the an atom first, followed by what is the name for the anion.
- If the cation is charged with more than one possible charge mark the charge in Roman numerals that are enclosed in parentheses.
- In the case of a multiatomic ion you should use the name given to the anion.
Examples:
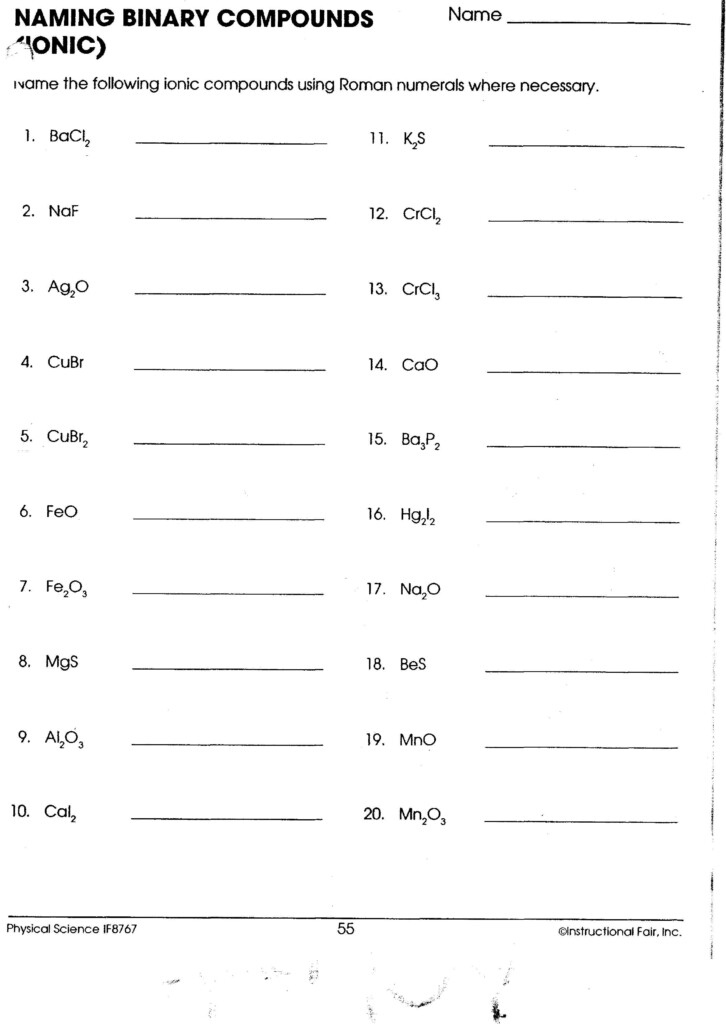
- NaCl is a synonym for sodium chloride.
- FeCl3 is also known as iron(III) chloride.
- Mg(NO3)2 is also known as magnesium oxide.
Naming Covalent Compounds
Covalent compounds arise from the sharing of electrons among atoms. They consist of molecules made consisting of two or even more atoms. The guidelines for naming compounds that are covalent are as like this:
- Write the name of the first element of the formula.
- Write“name” of second component in the formula, and change the ending in the form of “-ide”.
- Prefixes are used to indicate the quantity of atoms contained in each element in the molecule. Except for“mono,” for example “mono-” for the first element.
Examples:
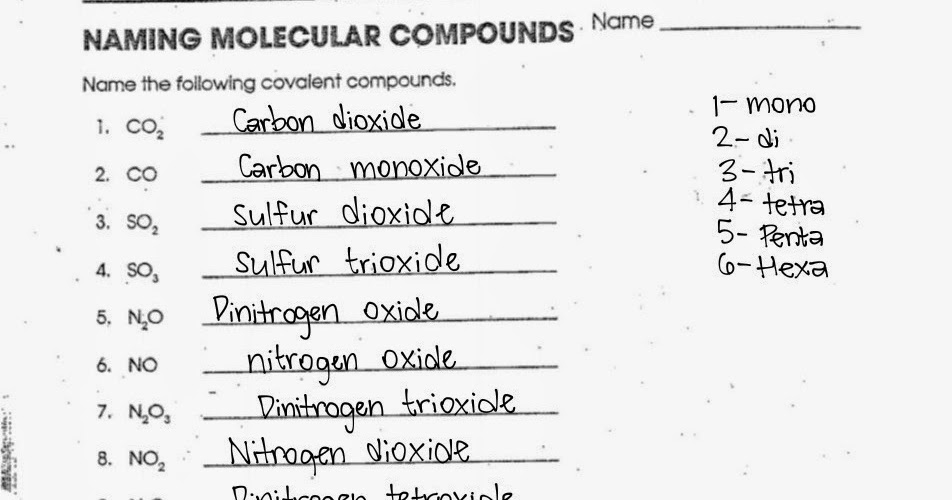
- Carbon dioxide is the name of CO2.
- N2O is named dinitrogen monoxide.
- This is known as sulfur hexafluoride.
Naming Binary Compounds
Binary compounds consist up of two elements. The rules for using the term binary compound are as they are:
- Write the name and the first element of the formula.
- Write an appropriate name for each element of the formula, and change the end to “-ide”.
Examples:
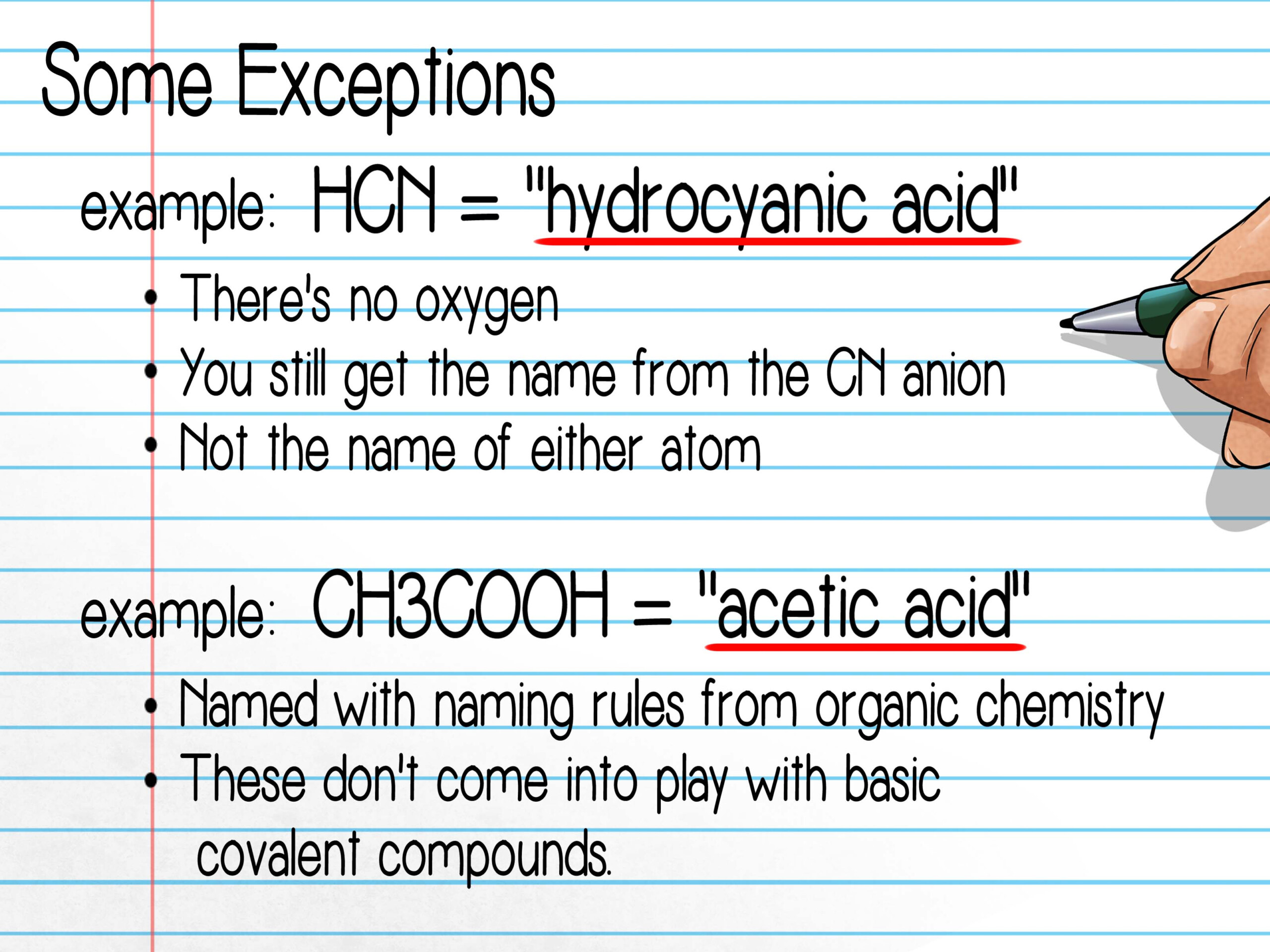
- The chemical name for HCl is hydrogen chloride.
- CO is named carbon monoxide.
- CaO is the name given to calcium oxide.
Practice Exercises
To strengthen the understanding it will be accompanied by drills for naming Ionic elements, covalent components, and binary compounds. These exercises will allow students to get a better understanding of the rules to name chemical compounds.
Ionic Compound Naming Exercises:
- Na2S
- KBr
- CaF2
- Al2O3
Covalent Compound Naming Exercises:
- CO
- SO2
- N2O4
- H2O2
Binary Compound Naming Exercises:
- Cl2O7
- P2S5
- BrF3
- NO
When they complete these activities, students will have confidence understanding chemical compound names and be able to apply these rules to other compounds.
Conclusion:
Naming compounds is an essential concept in chemistry . It demands a firm understanding what rules apply and the best practices for giving different compounds different names. Following the guidelines outlined in this worksheet and experimenting using the provided exercises, students can be confident in naming ionic, covalent, also binary compounds. This knowledge is vital to succeeding in chemistry and creates an excellent foundation for future studies in the area.