Review Naming Chemical Compounds Worksheet Answers – Naming compounds is a fundamental idea in chemical science. It involves assigning a unique name to any chemical compound based on its composition. When you name a compound gives important information about its properties as well as its structure. There are several kinds of chemical substances, including Ionic compounds, covalent substances, as well as binary compound.
Naming Ionic Compounds
Ionic compounds are formed by electron transfer between electrons. They consist in positively charged caustics and negatively charged anions. The rules to name ionic compounds are as follows:
- Inscribe the name of cation first, followed by it’s anion’s name.
- If the cation could have multiple possible charges then indicate the charge using Roman numerals in brackets.
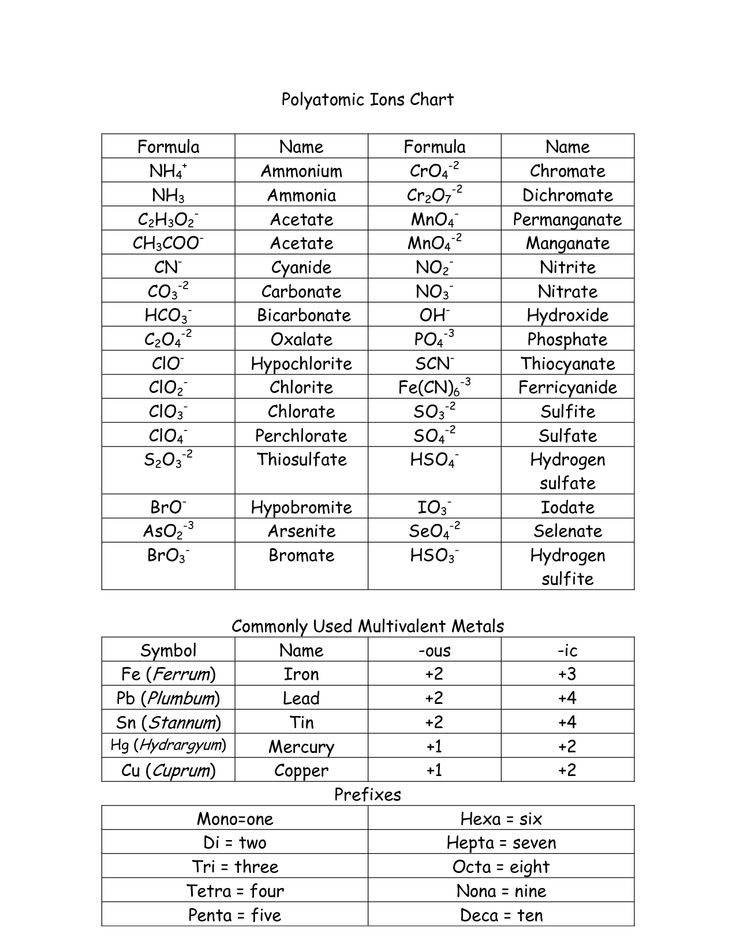
- The anion must be a polyatomic Ion, take the name of that ion.
Examples:
- NaCl is named sodium chloride.
- FeCl3 is also known as iron(III) chloride.
- Mg(NO3)2 is known as magnesium nurate.
Naming Covalent Compounds
Covalent compounds are formed by sharing electrons among atoms. They consist of molecules that are made comprised of two or three atoms. The guidelines for naming compounds that are covalent are as according to:
- Write the name of the first element of the formula.
- Enter the name of the second element in the formula, and change the end“ide “-ide”.
- Utilize prefixes to represent the number of elements in every element of the molecule. There is no prefix for“mono-,” the particular prefix “mono-” for the first element.
Examples:
- CO2 is the name given to carbon dioxide.
- N2O is named dinitrogen monoxide.
- So, SF6 is a sulfur hexafluoride.
Naming Binary Compounds
Binary compounds are substances made by two elements. The rules for calling binary compounds are as these:
- Inscribe the name of the first element of the formula.
- Write“double element” of the formula, changing the ending“ide “-ide”.
Examples:
- Hydrogen chloride is also known as hydrogen.
- CO is also known as carbon monoxide.
- CaO is the term used to describe calcium oxide.
Practice Exercises
To strengthen the understanding, the worksheet will include drills for naming Ionic elements, covalent components, in addition to binary compounds. This will help students improve their understanding of the rules that govern the naming of chemical compounds.
Ionic Compound Naming Exercises:
- Na2S
- KBr
- CaF2
- Al2O3
Covalent Compound Naming Exercises:
- CO
- SO2
- N2O4
- H2O2
Binary Compound Naming Exercises:
- Cl2O7
- P2S5
- BrF3
- NO
By finishing these exercises students will become more confident in making chemical compounds known and be able to apply the rules to other compounds.
Conclusion:
Naming compounds is an essential notion in chemistry and requires a deep understanding of these rules as well as guidelines to creating names for different kinds and types of compounds. By adhering to the guidelines set forth in this worksheet and experimenting through the exercises provided, students will be able comfortably identify covalent, ionic, and binary compounds. This is vital for success in chemistry . It also provides a strong foundation for further research in the field.